Hello! I'm Higgins, an undergraduate researcher specializing in organic synthesis! I spend much of my time synthesizing amines from tetralin-derived systems. I began my research journey in my Spring '24 semester of university. Since then, I have not regretted a single reaction, a single purification, or even a single second spent in lab. Synthesis is like an art, and I intend to paint the canvas until its flourishing with color.
Now, a little bit about my life outside of chemistry. In my freetime, I enjoy hiking, kayaking, programming, 3D modelling, environmental design (Source SDK) and simply spending time with my buddies. I also have a gato who I love very much!
Now let's get to the point. I intend this site to be used by any students, researchers or curious travelers who want to learn, or simply hear, some aspect of organic chemistry. Blogs will be posted about my funny, difficult and interesting lab experiences. I will also share advice, and lab tricks, I've personally found over the many reactions and troubleshooting encounters I've had. Lastly, I'd like to discuss a variety of chemistry concepts, synthetic reactions and even organic reagents I've handled (or wouldn't want to).
Thanks for reading!
In the realm of organic chemistry, the reactivity and identity of a chemical compound is defined by functional groups. But what are “functional groups?” Well, these groups are some unique, and recognizable, combination of atoms that behave in a defined manner. One group may increase a compound’s acidity, such as carboxylic acid (R-COOH), while others may strengthen its basicitiy, such as an imine (R=N-R). Many other prominent examples include ethers (R-O-R), thiols (R-SH), aldehydes (R-CH=O) and so forth.
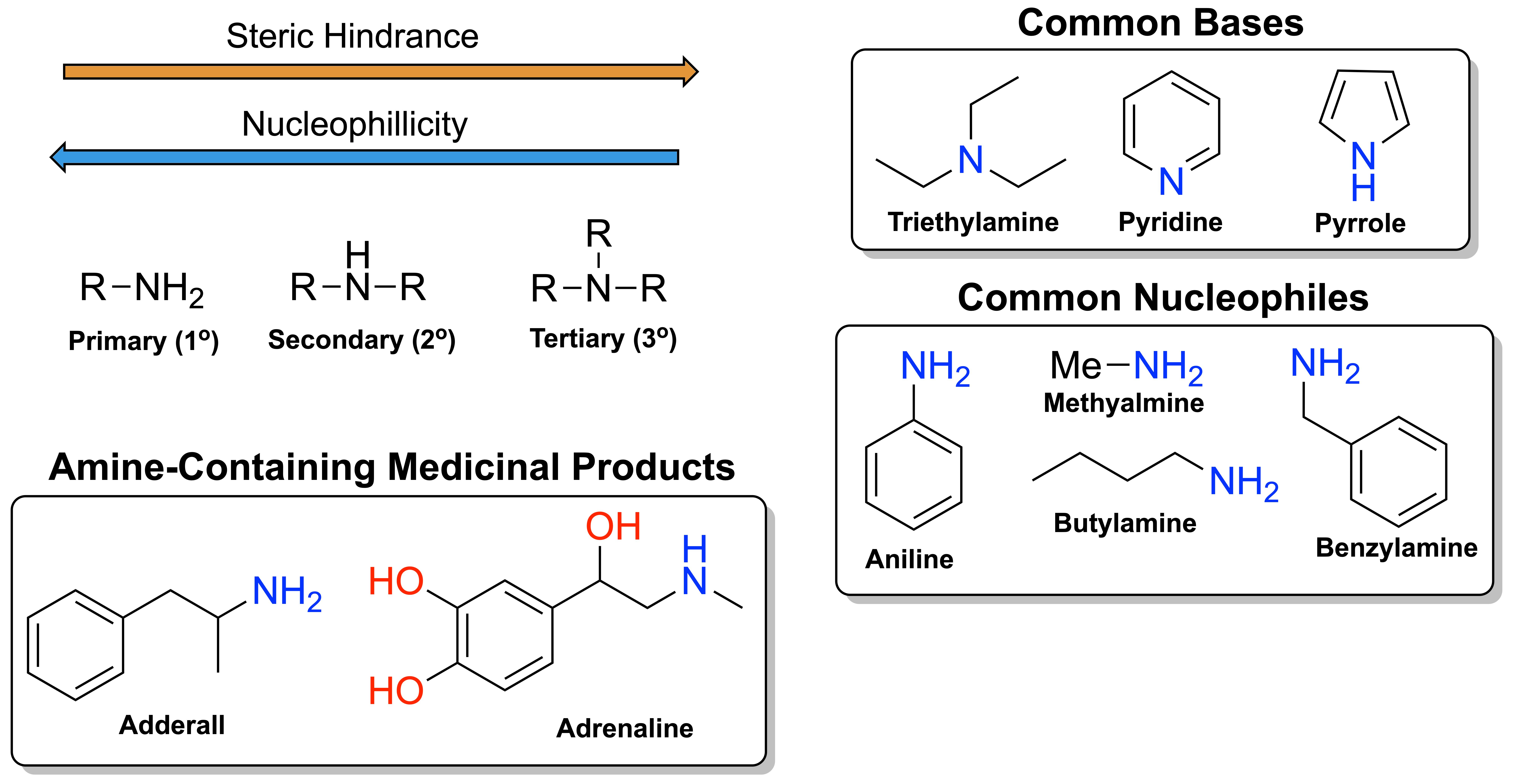
Within my specific research, amines present themselves as the most common functional group I personally encounter. What are these “amines” though, you haven’t mentioned them? I’d be happy to discuss that then. Amines are a nitrogen-centered functional group known for their applications in organic bases, medicinal compounds and biological molecules. They come in 3 classifications: primary (1o, R-NH2), secondary (2o, R-NH-R) and tertiary (3o, R2-N-R). Their property to act as a strong base, or nucleophile, is derived from the ready-to-donate lone pair on the nitrogen. This non-bonding electron pair is readily available to attack acidic hydrogens or electrophillic centers without a strong need to be activated by a base. Although amines suffer from overreacting in alkylation reactions and more, it's proven itself as both a powerful and versatile functional group in organic synthesis! There is so much to learn about amines...
Contact Information:
sonderamine@gmail.com